Wednesday, March 27, 2024
Monday, March 18, 2024
Monday, March 11, 2024
Saturday, March 9, 2024
Wednesday, March 6, 2024
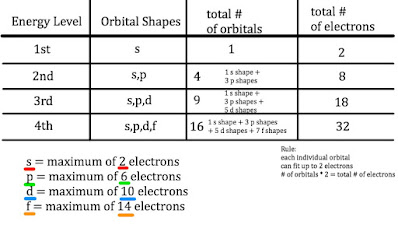
Orbital Energy Levels

Rules for filling orbitals... summarized:
Aufbau Principle: electrons fill from the lowest energy levels first.
*when filling in electrons, start at the bottom and work up.
Pauli Exclusion Principle: no two fermions can have the same quantum number.
*you can't have 2 electrons in the same place doing the same thing. Electrons can have spin up or spin down (this is 2 different quantum numbers). Therefore, if you have 2 electrons in 1 orbital, you must always have 1 up and 1 down. You may not have 2 ups or 2 downs. This is why we are limited to two electrons in each orbital.
Hunds rule: every orbital in a sublevel occupied by a single electron (going in the same spin direction) first before anything is doubly occupied.
*when given the choice, electrons choose to have their own orbital. Therefore, if there are 3 electrons in 3p, 1 will be in 3px, 1 in 3py, and 1 in 3pz.
Fill up each level with an up arrow on every line first before adding the second (down) arrow.

Monday, March 4, 2024
Bohr vs. Modern Atom
NEW PICTURE!
Quantized -- electrons must be within a certain energy level. They CANNOT be in between. This is why you see a bright line spectrum not a full spectrum.
What are these orbitals??
Numbers (1, 2, 3) tell you the energy level (similar to Bohr's Orbit Levels)
Letters (s, p, d, f) tell you the shape of the energy level (there are more options than rings/orbits/circles!)
Friday, March 1, 2024
Subscribe to:
Posts (Atom)